|
LaSOMI is a laboratory whose objective is the development and use of organic synthesis methodologies as an important tool in the preparation of new high performance organic materials applied in different branches of scientific and technological activity. These compounds involve a broad class of materials, from low molecular mass systems to polymeric structures. The preparation and the study of substances through the directed synthesis are of relevance, with wide application in the fields of organic electronic materials (OEM), with emphasis on liquid crystals (LC), organic light emitting diodes (OLEDs), organic molecules (transistors) that respond to the field effect (OFETs) and organic solar cells (OSCs). LaSOMI uses different organic synthesis methodologies in the preparation of new organic molecules. We emphasize on [3+2] 1,3-dipolar cycloaddition reactions from nitrile oxides to alkenes and alkynes, coupling reactions mediated by metal catalysts (Heck, Suzuki, Sonogashira, Ullman and Buchwald-Hartwig), oxidation and reduction reactions, esterification reactions via EDCI and DMAP, sigmatropic rearrangements of order [3,3], oximation, nucleophilic substitutions, etc. OEMs are intelligent materials capable of responding quickly when stimulated through some external effect, such as electrical, magnetic, light, pressure, heat, etc. The progress achieved in the field of organic synthesis combined with the available analytical techniques is the driving force for the development of intelligent materials in molecular, nano and mesomolecular regimes. Liquid crystals, gels, molecular probes, photo- and thermochromic compounds, (bio) chemical sensors, functionalized metallic nanoparticles, solar cells, elastomers and others form the set of intelligent materials whose properties can be modulated by the application of external stimuli and dependent on the intrinsic internal organization of the atoms, molecules or ions that constitute them.
Liquid Crystal
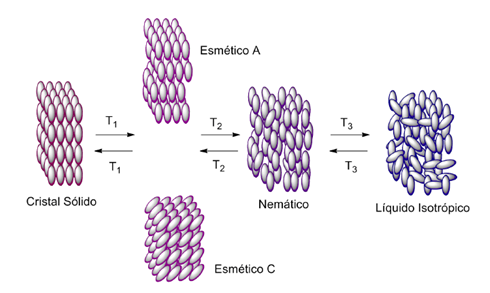
While a crystalline solid has positional and directional order of long distance in the three dimensions and a liquid does not present it, or presents at a very short distance, positional and directional order, a liquid crystal (LC) has an intermediate order between these two extremes. The compounds that show this form, add characteristics of both physical states, presenting both the organization and optical anisotropy of solids as well as fluid mobility (1). Due to this ambiguity, the liquid crystals present interesting and singular properties, nonexistent in other states of matter. For a better understanding of the liquid crystals, they are separated and classified into distinct groups. Thus, two major groups are described in the literature, thermotropic and lyotropic (1). Thermotropics describe the molecules that have, under certain temperatures, the formation of liquid-crystalline phases. Temperature is the determining factor that controls the appearance of these phases. As the liquid crystals are intermediates to the solid state and the liquid state, they are said to have one or more mesophases between these two states. The melting temperature is that which passes from the solid state to a mesophase, whereas the clearing temperature is the one that goes from the mesophase to the isotropic liquid.
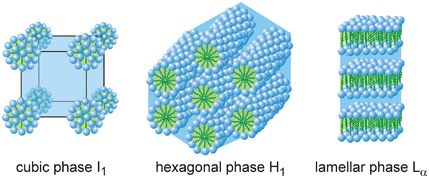
Lyotropics are formed by the addition of a solvent, which is commonly water (2). In addition, unlike thermotropics, the molecules are grouped into large sets called micelles. These aggregates follow the same behavior of surfactants, these molecules are characterized as amphiphilic. This means that one portion of the molecule is polar and another nonpolar portion. Molecules can also be arranged in layers or double layers (Figure 2).
In addition to the need of a solvent for the existence of liquid-crystalline properties, there is also a concentration limit called the critical micellar concentration (CMC). This value is usually in the range of 0.01 - 0.1 mass%. In general, a LC presents long-range orientational or positional order in at least one direction. In Figure 1 it is possible to visualize a sequence of thermal transitions for a calamithic thermotropic liquid crystal. In the liquid state there is no long-range orientational and/or positional order. In this condition, the molecules are arranged randomly in space, this state is said to be isotropic. By reducing the temperature, the loss of energy of the system favors the intermolecular interactions. In the simplest of all mesophases (N), there is only a middle orientation order for the molecules, they adopt a parallel alignment that minimizes the excluded volume and increases the intermolecular interactions. Nematic mesophase is the one that has characteristics closer to the liquid state, being more fluid than the other mesophases. With higher ordering (at lower temperatures) the smectic mesophases (Sm) are characterized by greater positional organization and less fluidity. The positional order means that the molecules begin to organize in layers (Figure 1). Although there is positional order due to the layers, the order of the molecules between layers can be variable, so that the smectic mesophases have different morphologies (3).
|

|
|||||
|
Contact us: Telephone: +55 51 3308-6293 UFRGS – Chemistry Institute Av. Bento Gonçalves 9500, Campus do Vale Building 43.131 (Building K), Laboratory K206 Bairro Agronomia – CEP 91501-970 Porto Alegre - RS
Phone: 3308-7316 - Room: K202 / Aloir A. Merlo Phone: 3308-7205 - Room: K209 / Renato Fax: +55 (51) 3308-7304 e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it. |